Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 13, Article number: 10143 (2023)
1389
3
6
Metrics details
Dog-infecting haemotropic mycoplasmas (haemoplasmas), such as Mycoplasma haemocanis and Candidatus Mycoplasma haematoparvum are common blood-borne pathogens of canines that can potentially inflict a substantial burden of disease, particularly in immunosuppressed individuals. Nonetheless, the transmission of these pathogens remains debated as more evidence emerges that they may not be transmitted by vectors, but instead use alternative methods such as aggressive interactions and vertical transmission. Here, we treated forty dogs with two different topically-acting ectoparasiticide products able to prevent vector-borne pathogen infections during an 8-month community trial in Cambodia. A total absence of ectoparasites were observed at all time points, and no new infections caused by pathogens confirmed as being vectorially-transmitted were detected, i.e., Babesia vogeli, Ehrlichia canis, Anaplasma platys, and Hepatozoon canis. Conversely, the number of haemoplasma infections in dogs on both ectoparasiticides rose significantly, with an incidence of 26 infections per 100 dogs at risk per year, providing strong evidence of non-vectorial transmission. Over the study period, dog aggression and fighting were frequently observed, highlighting a different potential mode of transmission. This study presents the first robust evidence that canine haemoplasmas may be transmitted without arthropod vectors drawing attention to the need for new methods to prevent their transmission.
Haemotropic mycoplasmas alternatively known as haemoplasmas are small epicellular bacterial pathogens, that typically reside on the surface of red blood cells1,2,3. These organisms lack a cell wall, are to date non-culturable and typically exhibit reduced genome sizes due to their parasitic existence4, 5. Haemotropic mycoplasmas can be relatively common blood-borne pathogens of canines that have been found across the globe, with species such as Mycoplasma haemocanis and Candidatus Mycoplasma haematoparvum being some of the most frequently detected2, 3, 6,7,8,9. These species can cause haemolytic anaemia which can range from a condition involving severe pathology, particularly in splenectomised or immunosuppressed dogs, through to a chronic condition with an absence of clinical signs that may go undetected3, 7, 10,11,12,13. In addition, the DNA of other haemotropic mycoplasmas has also been identified from dog blood, including Candidatus Mycoplasma turicensis14, 15, Candidatus Mycoplasma haemobos5, 9, Candidatus Mycoplasma haematominutum16 and an undescribed species infecting dogs in Australia and Cambodia12, 13, 17.
Importantly, some canine-infecting haemoplasma species have been found to cause infections in humans, e.g., C. M. haematoparvum which has been identified from a veterinarian exhibiting neurological symptoms including seizures and extreme headaches, raising questions on such species zoonotic potential18. Similarly, other zoonotic but non-canine haemotropic mycoplasma species have also been identified as generating human infections, for instance Candidatus Mycoplasma haemohominis19,20,21, a Mycoplasma ovis-like bacteria22, 23, and a Mycoplasma suis-like bacteria24. The clinical presentation in humans infected by these species is varied but in the case of C. M. haemohominis spans a range of symptoms including persistent pyrexia, haemolytic anaemia, and pancytopenia19, 20.
The transmission of canine haemotropic mycoplasmas remains a hotly debated topic that is exacerbated by them being unable to be axenically cultured1,2,3,4, 25. This characteristic of haemoplasmas consequently hampers researchers’ ability to conduct conclusive transmission studies and leads to a dearth in data on their basic biology2. Early investigations into canine haemotropic mycoplasmas suggested that transmission may be primarily facilitated using blood-feeding arthropods, such as the brown dog tick, Rhipicephalus sanguineus sensu lato26, more specifically the “tropical lineage” recently recognized as Rhipicephalus linnaei27, 28. Since then, other authors have highlighted vectors, including ticks and fleas, as likely taking a primary role in transmission7, 29. This is supported by more concrete evidence from studies exploring the transmission of the feline-infecting species Mycoplasma haemofelis, a close phylogenetic relative of M. haemocanis, where the role of Ctenocephalides felis fleas is strongly implicated in transmission30. Nevertheless, numerous studies have shown no association between the presence of arthropod vectors and haemoplasmas31,32,33 and other transmission modes have been reported, such as through blood transfusion34, vertical transmission from bitch-to-pup35, fighting, and social contact33, 36, 37. The detection of DNA from some feline haemoplasma species within salivary secretions of infected cats, highlights another mode by which these organisms may be transmitted37, 38. Therefore, whether multiple transmission modes are used by canine haemotropic mycoplasmas concurrently, or if different species use different forms of transmission, is a question that urgently needs addressing.
Here, we set out to assess whether two different topically-acting ectoparasiticide products would be able to prevent ectoparasite infestation and VBPs transmission during an 8-month long community trial conducted on 40 dogs in Cambodia. These topically-acting products act from the skin and fur of the animal to be quickly lethal and/or repel ectoparasites that might bite and transmit VBPs and elucidation of their efficacy in such a high VBP-transmission pressure context is crucial data for veterinarians and pet owners within the region39, 40.
Of the forty dogs investigated (cohort composition in Table 1) none were found with ectoparasites attached or present within either group at all timepoints, including baseline, demonstrating the efficacy of both Detick and Seresto® at preventing infestation by arthropod vectors.
Only qPCR was able to detect any of the identified blood-borne pathogens throughout this study with no infections found via microscopic examination of stained whole blood and buffy coat smears. The number of blood-borne pathogen infections detected via qPCR was highest at baseline (Table 2), i.e., prior to Detick and Seresto® application. Following on from baseline assessment the only newly acquired blood-borne pathogen infections were haemotropic mycoplasmas, with no other infections identified apart from one individual that remained Hepatozoon canis positive from baseline to the end of the study (Table 2). Given the challenges associated with H. canis curative treatment41 and because the dog did not show clinical signs throughout the study period, it was decided to withhold treatment, resulting in the subject continuing to remain positive till the end of the study period. When the number of infections for both groups of dogs were combined, no significant difference between haemotropic mycoplasma infections at baseline were observed, when compared to confirmed VBP infections, i.e., A. platys, E. canis and H. canis (χ2 test statistic 0.157, p = 0.69). However, after the application of either ectoparasiticide the number of newly acquired haemotropic mycoplasma infections was significantly higher than new infections by a pathogen known to be transmitted by vectors, at every time-point; 3-months (χ2 test statistic 5.000, p = 0.025), 6-months (χ2 test statistic 9.804, p = 0.002), and 8-months (χ2 test statistic 11.283, p = 0.001).
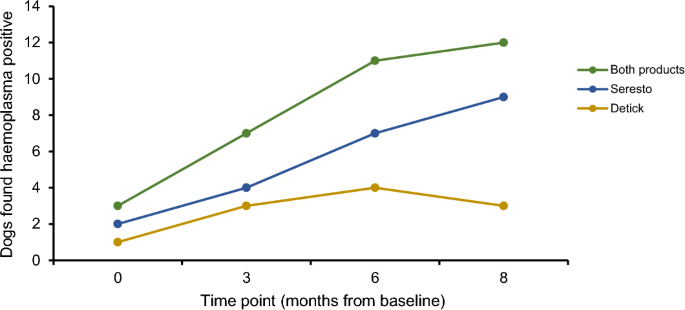
Haemotropic mycoplasma infections increased over the course of this trial, particularly in the Seresto® dog group (Fig. 1), whilst in the Detick group haemotropic mycoplasma infections increased from baseline to 3-months and then remained constant. The incidence of canine haemoplasma infection for dogs on Seresto® was 44 per 100 dogs at risk per year, whilst for Detick it was 11 per 100 dogs at risk per year, when the number of new haemoplasma infections was pooled for dogs on both ectoparasiticide products the incidence was 26 per 100 dogs at risk per year. Chi-squared tests found no difference between the number of haemotropic mycoplasma infections in dogs on Seresto® compared to those on Detick at all time points; baseline (χ2 test statistic 0.346, p = 0.556), 3-months (χ2 test statistic 0.157, p = 0.692), 6-months (χ2 test statistic 0.949, p = 0.330) and 8-months (χ2 test statistic 3.563, p = 0.059).
Change in the prevalence of haemoplasma infections in canines on the ectoparasiticides Detick, Seresto®, and the pooled results of dogs on both products, across an 8-month trial duration. Dots represent time points at which dogs were examined for ectoparasite presence and blood samples were collected and tested for pathogens.
When the haemoplasma results were pooled from dogs on both ectoparasiticide products, dog sex was found to influence infection status at the 8-month timepoint, with male dogs more likely to be haemoplasma positive than female dogs at this time point (χ2 test statistic 3.970, p = 0.046). However, variables such as dog neutering status (χ2 test statistic 1.119, p = 0.290) and whether a dog was above or below two years of age (χ2 test statistic 0.002, p = 0.964) were not found to impact whether an individual was haemoplasma positive at the 8-month timepoint.
Sanger sequencing of a fragment of the haemotropic mycoplasma RNAse P gene using blood samples from the dogs found positive via qPCR within this study, returned a top hit in GenBank with M. haemocanis (100% identity match and query cover with accession no. AF407211.1). NCBI accession numbers for our sequences are OQ378200 to OQ378206.
This community trial provided extensive information on the transmission of haemotropic mycoplasmas between dogs within closed environments in the absence of ticks, fleas, and lice. Whilst this study demonstrated that the ectoparasiticide products Seresto® and DeTick prevent canines from contracting confirmed VBPs, such as Babesia spp., A. platys, and E. canis, they did not prevent the contraction of haemoplasma infections in a country and local environment where these pathogens are highly endemic13. The large increase in haemoplasma infections detected over the course of this study demonstrates that both products are ineffective at preventing the transmission of such pathogens, likely due to them being communicable through mechanisms that are not reliant on a vector. Despite a report of vertical transmission from bitch to pup35 and other more circumstantial evidence of fighting-related transmission33, iatrogenic transmission through blood transfusion34 and a potential, but as yet unconfirmed role of arthropod vectors2, 7, 26, the mode(s) of canine haemotropic mycoplasma transmission is still not known. Here, we show clear evidence of non-vectorial canine haemoplasma transmission in the absence of vertical or transfusion-based infection, likely due to fighting between conspecifics in a closed environment. Prior data to indicate transmission of canine haemoplasmas via fighting has only been posited due to risk factor associations between dog aggression and haemotropic mycoplasma infection33, whilst this study builds upon such theories by providing more robust evidence.
The increase in haemotropic mycoplasma infections, particularly in the Seresto® group, could in our context be a result of aggressive behaviours as dogs were kept communally and free to move in large enclosures where fighting was observed to be commonplace. The owner and carer for these dogs reported regular instances of canine aggression and fighting between individuals. This violent behaviour was observed more regularly in the group of dogs on Seresto® (pers. comms. Georgia Kaczorowski, director of ‘House of Strays’), a fact that is further reflected in the breakage and removal of Seresto® collars in this group due to antagonistic interactions between dogs.
When the results for haemoplasma infection between dogs on both ectoparasiticides were pooled and assessed at the study’s end, i.e., the 8-month time point, male dogs were found to have significantly higher levels of haemoplasma infection than female dogs. Ethological research has highlighted differences in tendencies to display aggressive behaviours between the sexes in canines, with male dogs found to display more aggression, particularly towards conspecifics outside of their household and dogs of the same sex42,43,44. Such findings may support the hypothesis that canine haemotropic mycoplasmas transmission may be occurring through fluid exchanges as a result of biting and fighting within our cohort of dogs.
In contrast, no differences were found between haemoplasma infection and dog age category and neutering status, whilst the limited variety of dog breeds within our study group prevented comparison with this variable. The absence of difference in infection levels between canine neutering status is notable given that there has been some research suggesting intact dogs may demonstrate more aggressive behaviours and initiate more fights with conspecifics than neutered dogs43, 45, although, other studies have found no such association, or that the inverse is true46, 47. In Asia, the neutering status of dogs was strongly associated with exposure to a range of zoonotic parasites, however, a possible explanation for such an association is that owners who have their dogs neutered may have better access to veterinary care, as opposed to those that leave their dogs intact48.
Whilst specific research on the transmission of canine haemoplasmas is lacking, some relevant data can be gleaned from a larger body of research investigating feline haemotropic mycoplasmas. In cats infected with C. M. turicensis, this pathogen’s DNA was detected in the saliva and faeces of the host early in the course of infection38, whilst C. M. haemominutum DNA has been detected from the salivary glands of infected cats37. The presence of haemoplasmas in saliva and faeces could provide a means by which these pathogens are transmitted horizontally, either during fighting or through social contacts37, 38. In addition, Museux et al. created a laboratory model to replicate possible haemoplasma transmission events through cat fighting and found that when as little as 10 μl of cat blood infected with C. M. turicensis was inoculated subcutaneously into a naïve cat it could produce an infection49. The possibility of such transmission modes being employed by haemoplasma species that are closely related to canine hemotropic mycoplasmas poses the possibility that similar processes could be occurring for these pathogens.
No concomitant rises in pathogens known to be transmitted by arthropod vectors was observed within this study, whilst no ectoparasites were ever found on dogs in either group, further supporting our conclusion that the rise in canine haemoplasma infections was not due to vectorial transmission. Other studies have found similar results when looking for correlations between ectoparasite infestations and canine haemoplasma infection, garnering data that does not suggest a transmission role is being played by arthropod vectors31,32,33. The possibility that canine haemoplasmas use other vectors, such as mosquitoes or sandflies, that were not presently investigated is possible, although there is limited data suggesting such arthropods can act as viable vectors for these pathogens, whilst ectoparasiticides like Seresto® have been shown to be effective at preventing sandfly transmitted pathogens50,51,52.
Another putative explanation is that some dogs that tested positive for haemotropic mycoplasmas may have been pathogen carriers from the onset of the trial, whilst potentially fluctuating levels of parasitaemia meant that detection of these pathogens only occurred at certain time points. Such fluctuations in bacterial copy number, but no changes in the overall positivity status to haemoplasmas, have been observed before, in cats experimentally infected with C. M. turicensis49. This explanation could also potentially be the reason for the slight, but not statistically significant decrease in haemotropic mycoplasma infections observed from 6-months to 8-months in the group of dogs on Detick (one dog). Nonetheless, the sharp and statistically significant increase in the number of haemotropic mycoplasma positive dogs over time, together with a concomitant lack of detection of other VBPs and ectoparasites, strongly suggests that non-vectorial transmission of these pathogens is occurring.
Importantly, no treatment was initiated for haemotropic mycoplasmas until after the study had finished, hence the reduction in infections observed in the Detick group could either reflect a cure achieved by the dogs’ immune systems or a relative decrease in parasitaemia, making such infections temporarily undetectable by qPCR. However, the multiplex qPCR herein utilised has shown a very high analytical sensitivity of 5.2 × 10–4 fg/µl canine haemotropic mycoplasma DNA53, being suitable for the detection of animals with very low haemoplasma bacteraemia.
Environmental ectoparasite prevalence in Cambodia is known to be high with dogs on no ectoparasiticide product regularly found with tick and flea infestations, as well as high rates of VBP infection13. Therefore, the benefit and protection conferred by these ectoparasiticide products is great for those pathogens that are confirmed to be vectorially transmitted in countries such as Cambodia. Nonetheless, alternative measures need to be taken to prevent haemotropic mycoplasma infection, whilst further research on this group’s transmission is required to better unravel and understand the exact mechanism these pathogens harness to infect dogs. Our data is non-trivial given the potential pathogenesis canine haemotropic mycoplasmas can inflict on their hosts. Through cPCR and Sanger sequencing analysis we identified the haemoplasma species infecting our community dogs to be M. haemocanis which has been found to be highly prevalent in stray dogs from Cambodia before13. Species such as M. haemocanis and C. M. haematoparvum can generate a haemolytic anaemia with lethargy, fever, and icterus in infected dogs, whilst splenectomised or immunosuppressed individuals can show even more severe pathology that can lead to fatality11, 54,55,56. However, within this study no clinical abnormalities were observed between dogs infected versus those that were non-infected by canine haemoplasmas. In addition, some canine haemotropic mycoplasmas are zoonotic with one case in the literature identifying a C. M. haematoparvum, A. platys and Bartonella henselae coinfection from a symptomatic individual with pronounced disease18.
Overall, both Detick and Seresto® collars showed a very high efficacy at preventing ectoparasite infestation and VBP infection, however, both products failed to prevent haemoplasma infections, suggesting that these pathogens are transmitted between dogs through a mechanism that does not involve ectoparasite vectors. Future work to unequivocally demonstrate whether alternative transmission modes are used by dog haemoplasmas, such as through fighting or social contact, is urgently warranted to assist in the development of guidelines that will prevent canine infections from this pathogen group. This data will garner valuable basic biology data on haemotropic mycoplasmas and could in turn shed light on other pathogenic species that infect different hosts including animals and humans, that to date have unknown modes of transmission2, 19, 20, 25.
Ethical approval for this study was provided by the Office of Research Integrity and Ethics at the University of Melbourne, Australia, under ethics permit 1814620. This community trial was performed in accordance with the relevant guidelines and regulations including, but not limited to, those set by the University of Melbourne’s Office of Research Integrity and Ethics as well as those set by the Ministry of Agriculture, Forestry and Fisheries, Cambodia. Additionally, these methods are in accordance with ARRIVE guidelines.
This community trial was designed to compare the chemoprophylactic efficacy of two topical commercial acaricides; an imidacloprid 10% and flumethrin 4.5%, 8-month acting collar (Seresto®, Elanco) against a monthly spot-on containing [12% w/v] fipronil (Detick, Thailand), administered according to labelled instructions40. Both ectoparasiticide products act topically with the active compounds working from the subject’s skin or hair, hence they are designed to prevent vector feeding and reduce VBP transmission40.
A cohort of 40 dogs, with 20 in each treatment group (either Seresto® or Detick), were enrolled from the ‘House of Strays’ dog shelter in Siem Reap, Cambodia (13° 38′ N, 103° 85′ E), an organisation supported by the ‘Animals of Our World’ charity (registration no. 1197372). Animals were deemed eligible for enrolment if they were over eight weeks of age and were clinically normal on physical examination, as determined by a qualified veterinarian. The composition of dogs in both treatment groups was similar with regard to sex, age, and breed (Table 1), however the group given Seresto® had some individuals that had not been neutered (30%) whilst all dogs in the group given Detick were neutered. The only pure breed dog in our study cohort was a Pitbull.
All dogs had previously been administered the systemically-acting isoxazoline fluralaner as the formulation Bravecto® (Merck Animal Health), however they were only enrolled into this study after the window of labelled efficacy for this product had ceased. Enrolled dogs were housed as two closed and separate populations of canines at the ‘House of Strays’ shelter which consisted of two large outdoor enclosures in which the groups were kept separate and unable to intermingle. Nonetheless, dogs within each group could mix and interact as they wished within the specific section of the shelter they were located in, which comprised of large expanses of grass, sand/dirt and some tree cover. Dogs in each group were kept in their respective separate enclosures at night. Within the grounds of the shelter, dogs from both groups were regularly exposed to ectoparasites such as ticks, fleas, and lice that could enter the environment or be brought in from local stray dogs and/or new arrivals to the shelter.
This trial was conducted from 09.12.2020 to 20.08.2021 with Seresto® collars fitted and Detick applied at the start of trial. Seresto® has a purported 8-month duration of efficacy, hence these products did not need to be replaced over the course of the 8-month trial unless a collar was inadvertently removed during fighting or play which occurred in the case of eight dogs given these collars over the course of this study. If prematurely removed a new collar was applied within 48 h. For the Detick group, this ectoparasiticide was reapplied monthly between the shoulder blades as per the manufacturer’s instructions. Follow-up time points were at 3-, 6-, and 8-months after study commencement, when canine sampling and relevant metadata were collected.
At enrolment, dogs were subject to a complete physical examination by the study’s veterinarians. Each animal’s age, sex, breed, neutering status, whether imported, prior ectoparasite and endoparasite control history as well as other ongoing medication details were recorded. The presence or absence of ectoparasites was also assessed. Two millilitres of blood sample were drawn via cephalic or jugular venipuncture from each dog into two EDTA tubes. Blood was subjected to an examination for TBPs using stained whole blood and buffy coat smears. The second tube of whole blood was frozen at − 20 °C and transported on ice to the Melbourne Veterinary School at the University of Melbourne.
At the Melbourne Veterinary School DNA extraction on blood was conducted using a DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany) and subject to a previously developed multiplex real-time (qPCR) assay for common canine VBPs for the region53. Data was collected on the positivity status for the pathogens Anaplasma platys, Babesia gibsoni, Babesia vogeli, Ehrlichia canis, canine haemotropic mycoplasmas and Hepatozoon canis. Sample and metadata collection at follow up was then conducted at 3-, 6- and 8-months after trial commencement and at all time points qPCR on blood-extracted DNA was carried out.
At follow-up time points dogs were examined for the presence of ectoparasites for five minutes using visual observation and an ectoparasite comb with extra attention paid to common feeding sites of ticks e.g., head, ears, inter-digital regions. If ectoparasites were found, they were collected and preserved in 70% ethanol. Additionally, clinical signs consistent with VBP infection and potential adverse effects caused by their chemoprophylaxis treatment (e.g., dermatological changes at the site of application) were checked for. Individuals found positive to haemotropic mycoplasmas via qPCR during the trial were only treated at the end of the study i.e., after 8-months post commencement with a course of doxycycline 10 mg/kg SID for 28 days. For the individuals found positive to A. platys at the study’s baseline a 14-day course of doxycycline was initiated, whilst for the single E. canis positive dog found at baseline a 28-day course was commenced. No treatment was commenced for any dog found positive to a protozoan pathogen.
The utilised multiplex qPCR for canine VBP was only able to detect whether a sample was positive for canine haemotropic mycoplasmas at genus level. Hence, two conventional PCR (cPCR) assays were employed to provide species-level classification; one targeting a 600 bp stretch of the 16S ribosomal RNA (16S rRNA) gene of haemoplasmas57 and another targeting a 165 bp stretch of the haemotropic mycoplasma ribonuclease P gene (RNase P)58. All cPCRs were conducted using Taq 2X Master Mix (New England Biolabs, MA, USA) according to the published methods57, 58. Positive amplicons were cleaned using ExoSAP-IT™ PCR Product Cleanup Reagent (Thermo Fisher Scientific, MA, USA) and sent to Macrogen (Seoul, South Korea) for Sanger sequencing. Results were run through BLASTn, to compare them to existing GenBank reference sequences.
To identify differences between the number of newly acquired haemotropic mycoplasma infections between dogs on Detick and Seresto®, univariate analyses were conducted using 2 × 2 chi-square tests to assess whether there were statistically significant differences between the counts of haemoplasma qPCR test positives and negatives at all time points (p-value < 0.05). To identify associations between the number of newly acquired haemoplasma infections versus confirmed VBP infections, such as A. platys, E. canis and H. canis, 2 × 2 chi-square tests were also conducted, pooling infections from dogs on both ectoparasiticides together. Univariate analysis carried out via chi-squared tests were also used to assess canine haemoplasma infection status with dog sex, age, and neutering status at 8-months, irrespective of ectoparasiticide treatment.
Incidence rates for canine haemoplasma infection were determined by the number of new infections, i.e., the number of infections at 8-months minus the baseline infections, divided by the population at risk, i.e., total number of dogs that made it through the study minus those that were infected at baseline. In addition, the 95% confidence intervals (CIs) for haemotropic mycoplasma prevalence at all time points were calculated using the Wilson score interval via the open-source software Epitools (https://epitools.ausvet.com.au).
NCBI accession numbers for our Sanger sequences (Partial RNAse P gene of M. haemocanis) are OQ378200 to OQ378206. All datasheets, metadata and qPCR results are available from the authors upon request.
de Sousa, K. C. M. et al. Occurrence and molecular characterization of hemoplasmas in domestic dogs and wild mammals in a Brazilian wetland. Acta Trop. 171, 172–181 (2017).
Article PubMed Google Scholar
Millán, J., Di Cataldo, S., Volokhov, D. V. & Becker, D. J. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound. Emerg. Dis. 68, 3236–3256 (2020).
Article PubMed Google Scholar
Ravagnan, S. et al. Prevalence and molecular characterization of canine and feline hemotropic mycoplasmas (hemoplasmas) in northern Italy. Parasit. Vectors 10, 1–7 (2017).
Article Google Scholar
Biondo, A. W. et al. A review of the occurrence of hemoplasmas (hemotrophic mycoplasmas) in Brazil. Rev. Bras. Parasitol. Vet. 18, 1–7 (2009).
Article PubMed Google Scholar
Shi, H. et al. Molecular detection of haemophilic pathogens reveals evidence of Candidatus Mycoplasma haemobos in dogs and parasitic ticks in central China. BMC Vet. Res. 18, 1–8 (2022).
Article Google Scholar
Millán, J., Travaini, A., Cevidanes, A., Sacristán, I. & Rodríguez, A. Assessing the natural circulation of canine vector-borne pathogens in foxes, ticks and fleas in protected areas of Argentine Patagonia with negligible dog participation. Int. J. Parasitol. Parasites Wildl. 8, 63–70 (2019).
Article PubMed Google Scholar
Novacco, M. et al. Prevalence and geographical distribution of canine hemotropic mycoplasma infections in Mediterranean countries and analysis of risk factors for infection. Vet. Microbiol. 142, 276–284 (2010).
Article PubMed Google Scholar
Colella, V. et al. High-throughput microfluidic real-time PCR for the simultaneous detection of selected vector-borne pathogens in dogs in Bosnia and Herzegovina. Transbound. Emerg. Dis. 69, e2943–e2951 (2022).
Article CAS PubMed PubMed Central Google Scholar
Hii, S. F. et al. Canine vector-borne disease pathogens in dogs from south-east Queensland and north-east Northern Territory. Aust. Vet. J. 90, 130–135 (2012).
Article CAS PubMed Google Scholar
Kemming, G. et al. Can we continue research in splenectomized dogs? Mycoplasma haemocanis: Old problem—New insight. Eur. Surg. Res. 36, 198–205 (2004).
Article CAS PubMed Google Scholar
Di Cataldo, S. et al. Widespread infection with hemotropic mycoplasmas in free-ranging dogs and wild foxes across six bioclimatic regions of Chile. Microorganisms 9, 919 (2021).
Article PubMed PubMed Central Google Scholar
Huggins, L. G., Colella, V., Atapattu, A., Koehler, A. V. & Traub, R. J. Nanopore sequencing using the full length 16S rRNA gene for the detection of blood-borne bacteria in dogs reveals a novel species of haemotropic mycoplasma. Microbiol. Spectr. 21, e0308822 (2022).
Article Google Scholar
Huggins, L. G., Colella, V., Koehler, A. V., Schunack, B. & Traub, R. J. A multipronged next-generation sequencing metabarcoding approach unearths hyper-diverse and abundant dog pathogen communities in Cambodia. Transbound. Emerg. Dis. 69, 1933–1950 (2021).
Article PubMed Google Scholar
Huggins, L. G. et al. Assessment of a metabarcoding approach for the characterisation of vector-borne bacteria in canines from Bangkok, Thailand. Parasit. Vectors 12, 394 (2019).
Article PubMed PubMed Central Google Scholar
Soto, F. et al. Occurrence of canine hemotropic mycoplasmas in domestic dogs from urban and rural areas of the Valdivia Province, southern Chile. Comp. Immunol. Microbiol. Infect. Dis. 50, 70–77 (2017).
Article PubMed Google Scholar
Obara, H., Fujihara, M., Watanabe, Y., Ono, H. K. & Harasawa, R. A feline hemoplasma, ‘Candidatus Mycoplasma haemominutum’, detected in dog in Japan. J. Vet. Med. 73, 841–843 (2011).
Google Scholar
Barker, E. N. et al. Haemoparasites of free-roaming dogs associated with several remote Aboriginal communities in Australia. BMC Vet. Res. 8, 55 (2012).
Article PubMed PubMed Central Google Scholar
Maggi, R. G., Mascarelli, P. E., Havenga, L. N., Naidoo, V. & Breitschwerdt, E. B. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasit. Vectors 6, 1–10 (2013).
Article Google Scholar
Hattori, N. et al. Candidatus Mycoplasma haemohominis in human, Japan. Emerg. Infect. Dis. 26, 11 (2020).
Article CAS PubMed PubMed Central Google Scholar
Alcorn, K. et al. First report of Candidatus Mycoplasma haemohominis infection in Australia causing persistent fever in an animal carer. Clin. Infect. Dis. 72, 634–640 (2021).
Article PubMed Google Scholar
Steer, J. A. et al. A novel hemotropic mycoplasma (hemoplasma) in a patient with hemolytic anemia and pyrexia Clin. Infect. Dis. 53, e147–e151 (2011).
Article Google Scholar
Maggi, R. G. et al. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J. Clin. Microbiol. 51, 3237–3241 (2013).
Article PubMed PubMed Central Google Scholar
Sykes, J. E., Lindsay, L. A. L., Maggi, R. G. & Breitschwerdt, E. B. Human coinfection with Bartonella henselae and two hemotropic Mycoplasma variants resembling Mycoplasma ovis. J. Clin. Microbiol. 48, 3782–3785 (2010).
Article PubMed PubMed Central Google Scholar
Yuan, C. L. et al. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai. China. Am. J. Vet. Res. 70, 890–894 (2009).
Article CAS PubMed Google Scholar
Huggins, L. G., Koehler, A. V., Gasser, R. B. & Traub, R. J. Advanced approaches for the diagnosis and chemoprevention of canine vector-borne pathogens and parasites—Implications for the Asia-Pacific region and beyond. Adv. Parasitol. 118, 1–85 (2023).
Google Scholar
Seneviratna, P. & Weerasinghe & Ariyadasa, S.,. Transmission of Haemobartonella canis by the dog tick, Rhipicephalus sanguineus. Res. Vet. Sci. 14, 112–114 (1973).
Article CAS PubMed Google Scholar
Šlapeta, J., Chandra, S. & Halliday, B. The, “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). Int. J. Parasitol. 51, 431–436 (2021).
Article PubMed Google Scholar
Šlapeta, J., Halliday, B., Chandra, S., Alanazi, A. D. & Abdel-Shafy, S. Rhipicephalus linnaei (Audouin, 1826) recognised as the ‘tropical lineage’ of the brown dog tick Rhipicephalus sanguineus sensu lato: Neotype designation, redescription, and establishment of morphological and molecular reference. Ticks Tick Borne Dis. 13, (2022).
Willi, B. et al. Haemotropic mycoplasmas of cats and dogs: Transmission, diagnosis, prevalence and importance in Europe. Schweiz. Arch. 152, 237–244 (2010).
Article CAS Google Scholar
Woods, J. E., Brewer, M. M., Hawley, J. R., Wisnewski, N. & Lappin, M. R. Evaluation of experimental transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am. J. Vet. Res. 66, 1008–1012 (2005).
Article PubMed Google Scholar
Zarea, A. A. K. et al. Occurrence and bacterial loads of Bartonella and haemotropic Mycoplasma species in privately owned cats and dogs and their fleas from East and Southeast Asia. Zoonoses Public Health 69, 704–720 (2022).
Article PubMed PubMed Central Google Scholar
Barker, E. N. et al. Development and use of real-time PCR to detect and quantify Mycoplasma haemocanis and ‘Candidatus Mycoplasma haematoparvum’ in dogs. Vet. Microbiol. 140, 167–170 (2010).
Article ADS CAS PubMed PubMed Central Google Scholar
Cannon, S. H. et al. Infectious diseases in dogs rescued during dogfighting investigations. Vet. J. 211, 64–69 (2016).
Article CAS PubMed PubMed Central Google Scholar
Nury, C., Blais, M. C. & Arsenault, J. Risk of transmittable blood-borne pathogens in blood units from blood donor dogs in Canada. J. Vet. Intern. Med. 35, 1316–1324 (2021).
Article PubMed PubMed Central Google Scholar
Lashnits, E., Grant, S., Thomas, B., Qurollo, B. & Breitschwerdt, E. B. Evidence for vertical transmission of Mycoplasma haemocanis, but not Ehrlichia ewingii, in a dog. J. Vet. Intern. Med. 33, 1747–1752 (2019).
Article PubMed PubMed Central Google Scholar
Tasker, S. Haemotropic mycoplasmas: What’s their real significance in cats?. J. Feline Med. Surg. 12, 369–381 (2010).
Article PubMed PubMed Central Google Scholar
Dean, R. S., Helps, C. R., Gruffydd Jones, T. J. & Tasker, S. Use of real-time PCR to detect Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in the saliva and salivary glands of haemoplasma-infected cats. J. Feline Med. Surg. 10, 413–417 (2008).
Article PubMed Google Scholar
Willi, B. et al. Real-time PCR investigation of potential vectors, reservoirs, and shedding patterns of feline hemotropic mycoplasmas. Appl. Environ. Microbiol. 73, 3798–3802 (2007).
Article ADS CAS PubMed PubMed Central Google Scholar
Schorderet-Weber, S., Noack, S., Selzer, P. M. & Kaminsky, R. Blocking transmission of vector-borne diseases. Int. J. Parasitol. Drugs Drug Resist. 7, 90–109 (2017).
Article PubMed PubMed Central Google Scholar
Huggins, L. G. et al. Field trial investigating the efficacy of a long-acting imidacloprid 10%/flumethrin 4.5% polymer matrix collar (Seresto®, Elanco) compared to monthly topical fipronil for the chemoprevention of canine tick-borne pathogens in Cambodia. Curr. Res. Parasitol. Vector Borne Dis. 2, 100095 (2022).
Article CAS PubMed PubMed Central Google Scholar
Baneth, G. & Allen, K. Hepatozoonosis of dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 52, 1341–1358 (2022).
Reid, P. J., Cussen, V. A., Collins, K. A. & Lockwood, R. The utility of model dogs for assessing conspecific aggression in fighting dogs. Appl. Anim. Behav. Sci. 254, 105710 (2022).
Article Google Scholar
Sherman, C. K., Reisner, I. R., Taliaferro, L. A. & Houpt, K. A. Characteristics, treatment, and outcome of 99 cases of aggression between dogs. Appl. Anim. Behav. Sci. 47, 91–108 (1996).
Article Google Scholar
Borchelt, P. L. Aggressive behavior of dogs kept as companion animals: Classification and influence of sex, reproductive status and breed. Appl. Anim. Ethol. 10, 45–61 (1983).
Article Google Scholar
Beaver, B. V. Clinical classification of canine aggression. Appl. Anim. Ethol. 10, 35–43 (1983).
Article Google Scholar
Farhoody, P. et al. Aggression toward familiar people, strangers, and conspecifics in gonadectomized and intact dogs. Front. Vet. Sci. 5, 18 (2018).
Article PubMed PubMed Central Google Scholar
Guy, N. C. et al. Demographic and aggressive characteristics of dogs in a general veterinary caseload. Appl. Anim. Behav. Sci. 74, 15–28 (2001).
Article Google Scholar
Colella, V. et al. Human social conditions predict the risk of exposure to zoonotic parasites in companion animals in East and Southeast Asia. Commun. Med. 2, 144 (2022).
Article PubMed PubMed Central Google Scholar
Museux, K. et al. In vivo transmission studies of ‘Candidatus Mycoplasma turicensis’ in the domestic cat. Vet. Res. 40, 45 (2009).
Article PubMed PubMed Central Google Scholar
Reagan, K. L., Clarke, L. L., Hawley, J. R., Lin, P. & Lappin, M. R. Assessment of the ability of Aedes species mosquitoes to transmit feline Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’. J. Feline Med. Surg. 19, 798–802 (2016).
Article PubMed Google Scholar
Brianti, E. et al. Efficacy of a slow-release imidacloprid (10%)/flumethrin (4.5%) collar for the prevention of canine leishmaniosis. Parasit. Vectors 7, 327 (2014).
Article PubMed PubMed Central Google Scholar
Yimam, Y. & Mohebali, M. Effectiveness of insecticide-impregnated dog collars in reducing incidence rate of canine visceral leishmaniasis: A systematic review and meta-analysis. PLoS ONE 15, e0238601 (2020).
Article CAS PubMed PubMed Central Google Scholar
Huggins, L. G., Massetti, L., Schunack, B., Colella, V. & Traub, R. Novel high-throughput multiplex qPCRs for the detection of canine vector-borne pathogens in the Asia-Pacific. Microorganisms 9, 1092 (2021).
Article CAS PubMed PubMed Central Google Scholar
Cabello, J. et al. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): High prevalence and diversity of hemotrophic mycoplasmas. Vet. Microbiol. 167, 448–454 (2013).
Article PubMed Google Scholar
Pitorri, F. et al. Use of real-time quantitative PCR to document successful treatment of Mycoplasma haemocanis infection with doxycycline in a dog. Vet. Clin. Pathol. 41, 493–496 (2012).
Article PubMed Google Scholar
Torkan, S., Aldavood, S. J., Sekhavatmandi, A. & Moshkelani, S. Detection of haemotropic Mycoplasma (Haemobartonella) using multiplex PCR and its relationship with epidemiological factors in dogs. Comp. Clin. Pathol. 23, 669–672 (2014).
Article CAS Google Scholar
Criado-Fornelio, A., Martinez-Marcos, A., Buling-Saraña, A. & Barba-Carretero, J. C. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: A molecular study. Vet. Microbiol. 93, 307–317 (2003).
Article CAS PubMed Google Scholar
Maggi, R. G., Chitwood, M. C., Kennedy-Stoskopf, S. & DePerno, C. S. Novel hemotropic Mycoplasma species in white-tailed deer (Odocoileus virginianus). Comp. Immunol. Microbiol. Infect. Dis. 36, 607–611 (2013).
Article PubMed Google Scholar
Download references
The authors are greatly indebted to all employees at the ‘House of Strays’ animal refuge centre, Siem Reap, in particular the centre’s director Georgia Kaczorowski. Additionally, we would like to thank all the staff that participated in sample collection and project logistics for this research from Animal Mama Veterinary Hospital, Phnom Penh, and Cambodian Mine Action Centre (CMAC), Phnom Penh/Siem Reap.
This study was funded by an Australian Research Council Linkage grant LP170100187 with Elanco GmbH and Elanco Animal Health Australia as industry partners. Financial support was also provided by the University of Melbourne postgraduate scholarship scheme.
Melbourne Veterinary School, Faculty of Science, University of Melbourne, Parkville, VIC, 3050, Australia
Lucas G. Huggins, Rebecca J. Traub & Vito Colella
Animal Mama Veterinary Hospital, Phnom Penh, 12312, Cambodia
Zahida Baydoun, Ron Mab & Yulia Khouri
Elanco GmbH, Heinz-Lohmann-Str. 4, 27472, Cuxhaven, Germany
Bettina Schunack
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
L.G.H.; data curation, sample processing, project administration, formal analysis and validation, writing—original draft, reviewing & editing. Z.B.; sample collection and project administration. R.M.; sample collection. Y.K.; project administration and resources. B.S.; project administration and funding. R.J.T.; funding, administration, resources, writing—review and editing. V.C.; project conceptualization, project administration, formal analysis, writing—review and editing.
Correspondence to Lucas G. Huggins or Vito Colella.
Bettina Schunack is an employee of Elanco Animal Health. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
Huggins, L.G., Baydoun, Z., Mab, R. et al. Transmission of haemotropic mycoplasma in the absence of arthropod vectors within a closed population of dogs on ectoparasiticides. Sci Rep 13, 10143 (2023). https://doi.org/10.1038/s41598-023-37079-z
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37079-z
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Scientific Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Advertisement
© 2024 Springer Nature Limited
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.