Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 13, Article number: 8749 (2023)
8653
4
862
Metrics details
An Author Correction to this article was published on 03 July 2023
This article has been updated
Humans commit more violent crimes when temperature and air pollution is higher. Here, we investigate if also the day-to-day rates of dogs biting humans is influenced by environmental factors. 69,525 reports of dogs biting humans, sourced from public records on animal control requests and from ER records, were analyzed. The impact of temperature and air pollutants were evaluated with a zero-inflated Poisson generalized additive model, while controlling for regional and calendar effects. Exposure–response curves were used to assess the association between outcome and major exposure variables. We find that the rates of dogs biting humans increases with increasing temperature and ozone, but not PM2.5 exposure. We also observed that higher UV irradiation levels were related to higher rats of dog bites. We conclude that dogs, or the interactions between humans and dogs, are more hostile on hot, sunny, and smoggy days, indicating that the societal burden of extreme heat and air pollution also includes the costs of animal aggression.
Sarah Gähwiler, Annika Bremhorst, … Stefanie Riemer
Emma Hakanen, Salla Mikkola, … Hannes Lohi
Ana Maria Barcelos, Niko Kargas, … Daniel S. Mills
Aggression is a common behavior across species, with sometimes adaptive advantages to defending a territory, obtaining limited resources, competing for mates, or protecting members of the pack or tribe. Many acts of aggression may be conceptualized as the result of an imbalance between prefrontal “top-down” control systems and hyper-responsivity of limbic regions triggered by anger provoking stimuli1, a circuit that further appears modulated by striatal encoding of reward2,3,4. Human aggression has complex psychological and sociological roots, yet some external factors increase aggression across species: Higher temperature increases the likelihood of aggression among humans5,6,7, Rhesus monkeys8, rats9 and mice10. Inter-species aggression—dogs biting humans—has also been linked to higher temperatures11.
Short term exposure to air pollutants (particulate matter < 2.5 μm (PM2.5) and ozone)12,13,14,15,16,17,18 also appears to increase the incidence of human violent crime, as based on time-series analyses of air quality and criminal records data. It is not known if the link between air pollutants and aggression extends to other species.
To further investigate the link between air pollution exposure and aggression, we here explore public record of dogs biting humans. Dog bites represent 0.3% of all emergency department visit, and are a source of cosmetic disfigurement, trauma, finger amputation and occasional severe craniofacial injury and fatality19,20.
Multiple risk factors for dog bites have been identified, including dog specific factors (sex, castration/spay status, breed), victim factors (age, gender, familiarity with dog, victim behavior), and dog-victim interactions21,22,23,24,25.
The goal of this study was to determine potential environmental contributions to the daily prevalence of dog bites in 8 US cities during the years 2009 to 2018 in relation to temperature, the air pollutants PM2.5 and ozone, while controlling for precipitation, UV irradiation, calendar, and seasonal factors.
Dog bite incidents, typically recorded by city animal control authorities, were obtained from publicly available repositories for, Dallas26 and Houston27 (Texas), Baltimore (Maryland)28, Baton Rouge (Louisiana)29, Chicago (Illinois)30, Louisville31 (Kentucky) and New York City32 (New York). Data on dog bite incidents in Los Angeles23 (California) were compiled by Dr. Lisa Smith (Los Angeles County Department of Public Health) and Dr. Tony Kuo (University of California, Los Angeles) and used with permission. The above sources were selected because of availability and coverage, i.e., covering daily incidence over several years. As the included cities are of different size and used different reporting methods, we used the relative daily incidence in each city (daily incidence/city average daily incidence) as the outcome variable.
Daily counts of dog bites were zero-inflated (i.e., many days without incidents, Fig. S1 in the Appendix) and data was modeled utilizing a Poisson distribution in the ZIGAM model.
We obtained daily 24-h averages of PM2.5 (μg/m3) and daily 8-h maximum ozone (ppm) from the Environmental Protection Agency’s Air Quality System33 from all monitors within city limits. Average levels across all monitors were calculated for each city. We sourced precipitation and maximum daily temperatures from the National Oceanographic and Atmospheric Administration’s Climatology Network34. We sourced daily UV index for available cities from the National Weather Service Climate Prediction Center35. We excluded PM2.5 values more than 35 μg/m3 as there were few (0.12%) observations beyond this value.
To account for homogeneity of exposure effects, we standardized values of PM2.5, ozone, daily maximum temperature, precipitation, and UV index.
To estimate the association between day-to-day variations in exposure (PM2.5, ozone and temperature) on dog bite rates we applied a zero-inflated Poisson generalized additive (ZIGAM) model. We applied this model given that the daily counts of dog bites had many days without incidents (see Fig. S1 in the Appendix) and were therefore zero-inflated.
To adjust for potential confounding by seasonality and long term trend we included in the model penalized cubic splines of date per year of data. We also adjusted for federal holidays and weekends, and for cities with a categorical variable. The models included simultaneously daily PM2.5, ozone, maximum temperature, precipitation, and UV index. We ran multivariable models in a way where we have used date as non-linear term in the model and all the covariate as linear term to the model to accomplish the goal of create the exposure–response curve for each of the pollutants, separately. The exposure–response function (ERF) for each pollutant and temperature, in separate models, were calculated using a bootstrapping procedure: First we sample the data with replacement from the original data set. Then we apply the ZIGAM model on the bootstrapped data to build the model. Second, we then predict on the original data set at a given fixed value of the exposure variable, for example, PM2.5 on the range between the minimum and maximum value in the data set and averaged all predicted values to get an estimate of the ERF at that fixed value. Finally, we repeat Steps 1–2, for a large amount of time, to get all averaged ERF values at those given exposure levels. This way we get a bootstrapped version of the prediction model which considers the uncertainty of the original data and then since we are predicting on the original dataset, the distribution of covariates is set to be the same as in the original dataset. This approach allows us to estimate the ERF and also to compute the corresponding 95% confidence interval.
Initial graphical assessment on dog bites rates displayed some seasonality during the winter months in contrast to the non-winter months, as do other exposures. To examine whether the effects varied by winter and non-winter months we did stratified analysis by winter and non-winter months. In sensitivity analysis we examined whether the effect of ozone was confounded by UV index by excluding this variable from the model.
All hypothesis tests were two-sided and p values < 0.05 were considered statistically significant.
All analysis was performed using R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria (2020)). Main implementation of the ZIGAM model was run using the gam function of the mgcv R package.
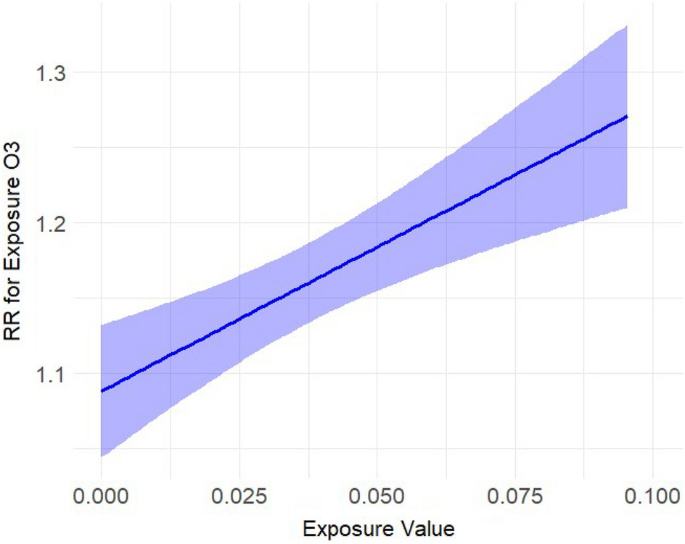
We included 11,082 complete datapoints, with a total of 69,525 reported dog bite incidents and an average three dog bites per day (Interquartile range (IQR) (one to eight incidents, see Supplement Table 1)), across 8 cities spanning 10 years. We find that dog bite incidence increased with increasing ozone (Fig. 1), temperature (Fig. 2), and UV irradiation, and decreased on rainy days and on holidays (see Table 1). There were however no effects of PM2.5 (Fig. 3). Results for ozone and UV irradiation remained significant when analyzing winter and non-winter months separately (Supplemental Table 3). As ozone levels covary with temperature and UV irradiation, in sensitivity analyses (see Supplement) we further modeled the results excluding either UV or ozone and found that the estimates of other variables were not influenced by either of them (see Supplement Table 2).
Estimated exposure–response curve for the exposure ozone (in ppm) on the rate of dog bites.
Estimated exposure–response curve for temperature (in Fahrenheit) on the rate of dog bites.
Estimated exposure–response curve for the exposure PM2.5 (in μg/m3) on the rate of dog bites.
Our results indicate that the daily incidence of dog bites is influenced by multiple environmental variables, including ozone, temperature, precipitation, and UV levels, but not PM2.5. Sensitivity analyses indicated that these relationships were stable and not greatly influenced by models or co-variance between variables. This is in line with prior studies on the impact of ozone on human aggression13,15,17,18, and studies on human aggression and temperature36. The effect of UV irradiation increasing aggression is in line with recent studies indicating increased aggression and increased sex-steroid levels after UVB exposure in mice and men37.
Ozone has a strong smell, is highly reactive and triggers oxidative stress in the airways and impairs pulmonary function. Due to its reactivity, ozone is not thought to penetrate beyond the membranes lining the respiratory tract and lungs, so behavioral effects may occur via generation of free radicals from lipid peroxidation. In humans, ozone exposure triggers the release of multiple messenger pathways, including serum amyloid A38, interleukin-639,40 and interleukin-841 and activation of the hypothalamic pituitary adrenal (HPA) axis42. Behavior may thus be influenced by a general stress response to pollutants triggered by lung inflammatory messengers. More direct effects on brain function are also possible: In rats, acute ozone exposure rapidly increases dopamine43, noradrenaline, dihydroxyphenylacetic acid, and 5-hydroxyindolacetic acid in the striatum and midbrain44. Ozone exposure further stimulates catecholamine biosynthesis in the hindbrain noradrenergic A2 group, catecholamine turnover is increased in the cortex, but decreased in the striatum45. In human experimental ozone exposure studies, 4 h of 200 ppb ozone exposure led to a 79% increase in 8-isoprostane (8-ISO), a measure of lipid oxidation, 18 h after exposure46. Notably, 8-ISO levels are elevated in intermittent explosive disorder, and further correlated to measures of actual aggressive behaviors47. As the neural circuitry for aggressive behaviors is conserved across mammals and given the impact of ozone on basal ganglia dopaminergic function, we speculate that ozone may influence aggressive behavior via impacts on dopamine turnover in the striatum. While combustion derived PM2.5 has been detected in the brains of both dogs48 and humans49, we did not observe and effect of PM2.5 on dog bite incidence. Compared to humans, dogs have a much larger surface area of olfactory epithelium, more olfactory receptors, and a larger olfactory bulb50,51. As such, anatomical differences between humans and dogs may account for the lack of effect in this study.
We utilized animal control and hospital records to evaluate the impact of temperature and air pollutants on dog bite incidence. However, survey data indicates that the true burden of dog bites is much higher than reported in hospital data52 and only a small percentage of dog bites require extensive medical treatment or hospitalization53. Our results are therefore likely indicative of more severe dog bite incidents. According to prior studies, most dog bites arise from a dog known to the victim, and most bites are related to interacting or attempting to interact with the dog21,25. While it is likely that human–dog interactions increase on days with higher temperature and higher UV irradiation (i.e., sunny days), our analysis indicates that ozone levels further contribute to the risk of dog bites, an effect present in both winter and summer months independently. Moreover, our analysis indicates a slight decreased risk on weekends and holidays, suggesting that ample time for dog–human interactions does not increase risk.
A limitation of our analysis is that public records of dog bites do not provide more detailed information about dog breed, sex, castration/spaying status, nor for bite severity, victim age, gender, familiarity with dog and the interactions leading up to the dog bite, all factors that impact the risk and consequence of dog bites21,23,24,25.
We included data spanning 2009 to 2019. Earlier datapoints were not publicly available from our sources. We did not include data from the COVID-19 era. During COVID-19 lockdowns, air pollution decreased, but pediatric emergency department visits for dog bites increased54,55. This suggest that other factors, such as forced proximity, may be a larger determinant in dog-on-human aggression. According to the American Veterinary Medicine Association, dogs bite primarily as a reaction to something, such as stressful situations, a scare, startle, or threat, or to protect food, toys or their puppies56. Dogs might bite defensively or to be left alone22,24,25. In our analysis, it is unclear if dog behavior is directly altered by ozone and heat, or, if the observed increase in dog bites is a consequence of altered behavior imposed by the human victim and/or the dogs master, which in many cases are the same individual21.
The effects of increasing temperature and air pollutants on human aggression, as indexed by police records, are well established5,6,7,12,13,14,15,16,17. Yet police records of criminal activity, while extensive and well documented, may have systematic biases: less than 45% of violent crimes are reported to law enforcement57. Criminal reporting may further be impacted by the behavior of victims and bystanders, as well as by the priorities and resources of law enforcement. The present findings, expand the association between temperature, air pollutants and aggression across species to also include dogs. It is notable that in rodents, exposure to ozone, heat stress, and their combination induces cognitive decline and neuroinflammation58. The link between ozone and aggression awaits verification such as by randomized double blinded exposure experiments in animals or possibly humans. While cardiovascular and pulmonary health effects of pollution are well established, the present results emphases the impacts on behavior and mental health. Through such mechanism, air pollutants and extreme heat could contribute to higher societal and individual burdens then currently appreciated.
All data was obtained from public repositories as referenced, except dog bite incidents in Los Angeles23 (California) which were compiled by Dr. Lisa Smith (Los Angeles County Department of Public Health) and Dr. Tony Kuo (University of California, Los Angeles) and used with permission. The curated datasets generated and analyzed during the current study are available from the corresponding author on reasonable request, with data from Los Angeles also contingent on original author permission23.
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-37841-3
Rosell, D. R. & Siever, L. J. The neurobiology of aggression and violence. CNS Spectr. 20, 254–279. https://doi.org/10.1017/S109285291500019X (2015).
Article PubMed Google Scholar
Glenn, A. L. & Yang, Y. The potential role of the striatum in antisocial behavior and psychopathy. Biol. Psychiat. 72, 817–822. https://doi.org/10.1016/j.biopsych.2012.04.027 (2012).
Article PubMed Google Scholar
Golden, S. A. et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. https://doi.org/10.1038/nature18601 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Chester, D. S. & DeWall, C. N. The pleasure of revenge: Retaliatory aggression arises from a neural imbalance toward reward. Soc. Cogn. Affect. Neurosci. 11, 1173–1182. https://doi.org/10.1093/scan/nsv082 (2016).
Article PubMed Google Scholar
Berman, J. D., Bayham, J. & Burkhardt, J. Hot under the collar: A 14-year association between temperature and violent behavior across 436 U.S. counties. Environ. Res. 191, 110181. https://doi.org/10.1016/j.envres.2020.110181 (2020).
Article CAS PubMed Google Scholar
Cohn, E. G. & Rotton, J. The curve is still out there: A reply to Bushman, Wang, and Anderson’s (2005) “Is the curve relating temperature to aggression linear or curvilinear?”. J. Pers. Soc. Psychol. 89, 67–70. https://doi.org/10.1037/0022-3514.89.1.67 (2005).
Article PubMed Google Scholar
Stevens, H. R., Beggs, P. J., Graham, P. L. & Chang, H. C. Hot and bothered? Associations between temperature and crime in Australia. Int. J. Biometeorol. 63, 747–762. https://doi.org/10.1007/s00484-019-01689-y (2019).
Article ADS PubMed Google Scholar
Xu, A., Liu, C., Wan, Y., Bai, Y. & Li, Z. Monkeys fight more in polluted air. Sci. Rep. 11, 654. https://doi.org/10.1038/s41598-020-80002-z (2021).
Article CAS PubMed PubMed Central Google Scholar
Berry, R. M. & Jack, C. E. The effect of temperature upon shock-elicited aggression in rats. Psychon. Sci. 23, 341–342. https://doi.org/10.3758/BF03336141 (1971).
Article Google Scholar
Greenberg, G. The effects of ambient temperature and population density on aggression in two inbred strains of mice, Mus Musculus. Behaviour 42, 119–130. https://doi.org/10.1163/156853972X00130 (1972).
Article CAS PubMed Google Scholar
Zhang, Y. et al. Are hospital emergency department visits due to dog bites associated with ambient temperature? A time-series study in Beijing, China. Sci. Total Environ 598, 71–76. https://doi.org/10.1016/j.scitotenv.2017.04.112 (2017).
Article ADS CAS PubMed Google Scholar
Berman, J. D., Burkhardt, J., Bayham, J., Carter, E. & Wilson, A. Acute air pollution exposure and the risk of violent behavior in the United States. Epidemiology 30, 799–806. https://doi.org/10.1097/EDE.0000000000001085 (2019).
Article PubMed Google Scholar
Burkhardt, J. et al. The effect of pollution on crime: Evidence from data on particulate matter and ozone. J. Environ. Econ. Manag. 98, 102267. https://doi.org/10.1016/j.jeem.2019.102267 (2019).
Article Google Scholar
Burkhardt, J. et al. The relationship between monthly air pollution and violent crime across the United States. J. Environ. Econ. Policy https://doi.org/10.1080/21606544.2019.1630014 (2019).
Article Google Scholar
Lu, J. G., Lee, J. J., Gino, F. & Galinsky, A. D. Polluted morality: Air pollution predicts criminal activity and unethical behavior. Psychol. Sci. 29, 340–355. https://doi.org/10.1177/0956797617735807 (2018).
Article PubMed Google Scholar
Bondy, M., Roth, S. & Sager, L. Crime is in the Air: The Contemporaneous Relationship between Air Pollution and Crime. IDEAS Working Paper Series from RePEc (2018).
Herrnstadt, E. & Muehlegger, E. Air pollution and criminal activity: Evidence from Chicago microdata. NBER Working Paper Series, 21787. https://doi.org/10.3386/w21787 (2015).
Rotton, J. & Frey, J. Air pollution, weather, and violent crimes: Concomitant time-series analysis of archival data. J Pers Soc Psychol 49, 1207–1220. https://doi.org/10.1037//0022-3514.49.5.1207 (1985).
Article CAS PubMed Google Scholar
Ramgopal, S. & Macy, M. L. US estimates for dog bite injuries presenting to emergency departments. Public Health 196, 1–3. https://doi.org/10.1016/j.puhe.2021.04.028 (2021).
Article CAS PubMed Google Scholar
Tuckel, P. S. & Milczarski, W. The changing epidemiology of dog bite injuries in the United States, 2005–2018. Inj Epidemiol. 7, 57. https://doi.org/10.1186/s40621-020-00281-y (2020).
Article PubMed PubMed Central Google Scholar
Barrios, C. L. et al. Epidemiology of dog bite incidents in Chile: Factors related to the patterns of human-dog relationship. Animals 11, 1–96. https://doi.org/10.3390/ani11010096 (2021).
Article Google Scholar
Haug, L. I. Canine aggression toward unfamiliar people and dogs. Vet. Clin. North Am. Small Anim. Pract. 38, 1023–1041. https://doi.org/10.1016/j.cvsm.2008.04.005 (2008).
Article PubMed Google Scholar
Lyu, C. et al. Burden of bites by dogs and other animals in Los Angeles County, California, 2009–2011. Public Health Rep. 131, 800–808. https://doi.org/10.1177/0033354916675148 (2016).
Article PubMed PubMed Central Google Scholar
O’Sullivan, E. N., Jones, B. R., O’Sullivan, K. & Hanlon, A. J. Characteristics of 234 dog bite incidents in Ireland during 2004 and 2005. Vet. Rec. 163, 37–42. https://doi.org/10.1136/vr.163.2.37 (2008).
Article CAS PubMed Google Scholar
Oxley, J. A., Christley, R. & Westgarth, C. Contexts and consequences of dog bite incidents. J. Vet. Behav. 23, 33–39. https://doi.org/10.1016/j.jveb.2017.10.005 (2018).
Article Google Scholar
Dallas Open Data DAS Field Data. https://www.dallasopendata.com/City-Services/FY2019-Dallas-Animals-Field-Data/rgsr-utra
Open Data Houston BARC Animal Service Requests. http://data.houstontx.gov/dataset/barc-animal-service-requests
Open Baltimore 311 Animal Issues. https://data.baltimorecity.gov/datasets/baltimore::311-customer-service-requests-2020/about.
Open Data BR Animal Conrol Incidents. https://data.brla.gov/Public-Safety/Animal-Control-Incidents/qmns-hw3s
City of Chicago Animal Care and Control. https://www.chicago.gov/city/en/depts/cacc.html
Louisville Metro Open Data. https://data.louisvilleky.gov/datasets/LOJIC::louisville-metro-ky-animal-services-activity-log/explore.
NYC Open Data dohmh Dog Bite Data. https://data.cityofnewyork.us/Health/DOHMH-Dog-Bite-Data/rsgh-akpg
Environmental Protection Agencys Air Quality System. https://www.epa.gov/outdoor-air-quality-data/download-daily-data
National Oceanographic and Atmospheric Administration’s Climatology Network. https://www.ncdc.noaa.gov/data-access/quick-links#ghcn
National Weather Service Climate Prediction Center. https://www.cpc.ncep.noaa.gov/products/stratosphere/uv_index/uv_annual.shtml
Anderson, C. A., Anderson, K. B., Dorr, N., DeNeve, K. M. & Flanagan, M. Advances in Experimental Social Psychology Vol. 32, 63–133 (Academic Press, 2000).
Google Scholar
Parikh, R. et al. Skin exposure to UVB light induces a skin-brain-gonad axis and sexual behavior. Cell Rep. 36, 109579. https://doi.org/10.1016/j.celrep.2021.109579 (2021).
Article CAS PubMed PubMed Central Google Scholar
Erickson, M. A. et al. Serum amyloid A: An ozone-induced circulating factor with potentially important functions in the lung-brain axis. FASEB J. 31, 3950–3965. https://doi.org/10.1096/fj.201600857RRR (2017).
Article CAS PubMed PubMed Central Google Scholar
Bennett, W. D. et al. Effect of obesity on acute ozone-induced changes in airway function, reactivity, and inflammation in adult females. PLoS ONE 11, e0160030. https://doi.org/10.1371/journal.pone.0160030 (2016).
Article CAS PubMed PubMed Central Google Scholar
Thompson, A. M. et al. Baseline repeated measures from controlled human exposure studies: Associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ. Health Perspect. 118, 120–124. https://doi.org/10.1289/ehp.0900550 (2010).
Article CAS PubMed Google Scholar
Devlin, R. B. et al. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126, 104–111. https://doi.org/10.1161/CIRCULATIONAHA.112.094359 (2012).
Article CAS PubMed Google Scholar
Miller, D. B. et al. Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am. J. Respir. Crit. Care Med. 193, 1382–1391. https://doi.org/10.1164/rccm.201508-1599OC (2016).
Article CAS PubMed PubMed Central Google Scholar
Rivas-Arancibia, S. et al. Effect of acute ozone exposure on locomotor behavior and striatal function. Pharmacol. Biochem. Behav. 74, 891–900. https://doi.org/10.1016/s0091-3057(03)00011-x (2003).
Article CAS PubMed Google Scholar
Gonzalez-Pina, R. & Paz, C. Brain monoamine changes in rats after short periods of ozone exposure. Neurochem. Res. 22, 63–66. https://doi.org/10.1023/a:1027329405112 (1997).
Article CAS PubMed Google Scholar
Soulage, C. et al. Central and peripheral changes in catecholamine biosynthesis and turnover in rats after a short period of ozone exposure. Neurochem. Int. 45, 979–986. https://doi.org/10.1016/j.neuint.2004.06.015 (2004).
Article CAS PubMed Google Scholar
Chen, C., Arjomandi, M., Balmes, J., Tager, I. & Holland, N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ. Health Perspect. 115, 1732–1737. https://doi.org/10.1289/ehp.10294 (2007).
Article CAS PubMed PubMed Central Google Scholar
Coccaro, E. F., Lee, R. & Gozal, D. Elevated plasma oxidative stress markers in individuals with intermittent explosive disorder and correlation with aggression in humans. Biol. Psychiat. 79, 127–135. https://doi.org/10.1016/j.biopsych.2014.01.014 (2016).
Article CAS PubMed Google Scholar
Calderon-Garciduenas, L. et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 68, 117–127. https://doi.org/10.1016/j.bandc.2008.04.008 (2008).
Article PubMed Google Scholar
Gonzalez-Maciel, A., Reynoso-Robles, R., Torres-Jardon, R., Mukherjee, P. S. & Calderon-Garciduenas, L. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: Culprit hidden in plain sight in Alzheimer’s disease development. J. Alzheimers Dis. 59, 189–208. https://doi.org/10.3233/JAD-170012 (2017).
Article CAS PubMed Google Scholar
Jenkins, E. K., DeChant, M. T. & Perry, E. B. When the nose doesn’t know: Canine olfactory function associated with health, management, and potential links to microbiota. Front. Vet. Sci. 5, 56. https://doi.org/10.3389/fvets.2018.00056 (2018).
Article PubMed PubMed Central Google Scholar
Craven, B. A. et al. Reconstruction and morphometric analysis of the nasal airway of the dog (Canis familiaris) and implications regarding olfactory airflow. Anat. Rec. (Hoboken) 290, 1325–1340. https://doi.org/10.1002/ar.20592 (2007).
Article PubMed Google Scholar
Westgarth, C., Brooke, M. & Christley, R. M. How many people have been bitten by dogs? A cross-sectional survey of prevalence, incidence and factors associated with dog bites in a UK community. J. Epidemiol. Community Health 72, 331–336. https://doi.org/10.1136/jech-2017-209330 (2018).
Article PubMed Google Scholar
Holzer, K. J., Vaughn, M. G. & Murugan, V. Dog bite injuries in the USA: Prevalence, correlates and recent trends. Inj Prev. 25, 187–190. https://doi.org/10.1136/injuryprev-2018-042890 (2019).
Article PubMed Google Scholar
Sethuraman, U. et al. Trauma visits to a pediatric emergency department during the COVID-19 quarantine and “Stay at Home” period. Am. Surg. https://doi.org/10.1177/00031348211047497 (2021).
Article PubMed Google Scholar
Dixon, C. A. & Mistry, R. D. Dog bites in children surge during coronavirus disease-2019: A case for enhanced prevention. J. Pediatr. 225, 231–232. https://doi.org/10.1016/j.jpeds.2020.06.071 (2020).
Article CAS PubMed PubMed Central Google Scholar
AVMA. Dog Bite Prevention, https://www.avma.org/resources-tools/pet-owners/dog-bite-prevention (2023).
Morgan, R. E. & Oudekerk, B. A. U.S. Department of Justice Criminal Victimization, 2018, https://www.bjs.gov/content/pub/pdf/cv18.pdf (2019).
Yan, Z., Liu, Y. M., Wu, W. D., Jiang, Y. & Zhuo, L. B. Combined exposure of heat stress and ozone enhanced cognitive impairment via neuroinflammation and blood brain barrier disruption in male rats. Sci. Total Environ. 857, 15959. https://doi.org/10.1016/j.scitotenv.2022.159599 (2023).
Article CAS Google Scholar
Download references
This study was funded by NIH Grants 1R01AG066793 and ES000002.
Department of Surgery, Center for Surgery and Public Health, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Tanujit Dey
Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Antonella Zanobetti
Spaulding Neuroimaging Laboratory, Department of PM&R, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, MA, USA
Clas Linnman
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
T.D. analyzed data, prepared figures, and contributed to the manuscript. A.Z. contributed to study concept, data analysis and the manuscript C.L. conceptualized study, collected data, analyzed data and wrote the main manuscript. All authors reviewed the manuscript.
Correspondence to Clas Linnman.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 2, which was incorrectly given as ‘Department of Environmental Health, Harvard School of Public Health, Boston, MA, USA.’ The correct affiliation is: Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
Dey, T., Zanobetti, A. & Linnman, C. The risk of being bitten by a dog is higher on hot, sunny, and smoggy days. Sci Rep 13, 8749 (2023). https://doi.org/10.1038/s41598-023-35115-6
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35115-6
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Ambio (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Collection
Advertisement
© 2024 Springer Nature Limited
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.